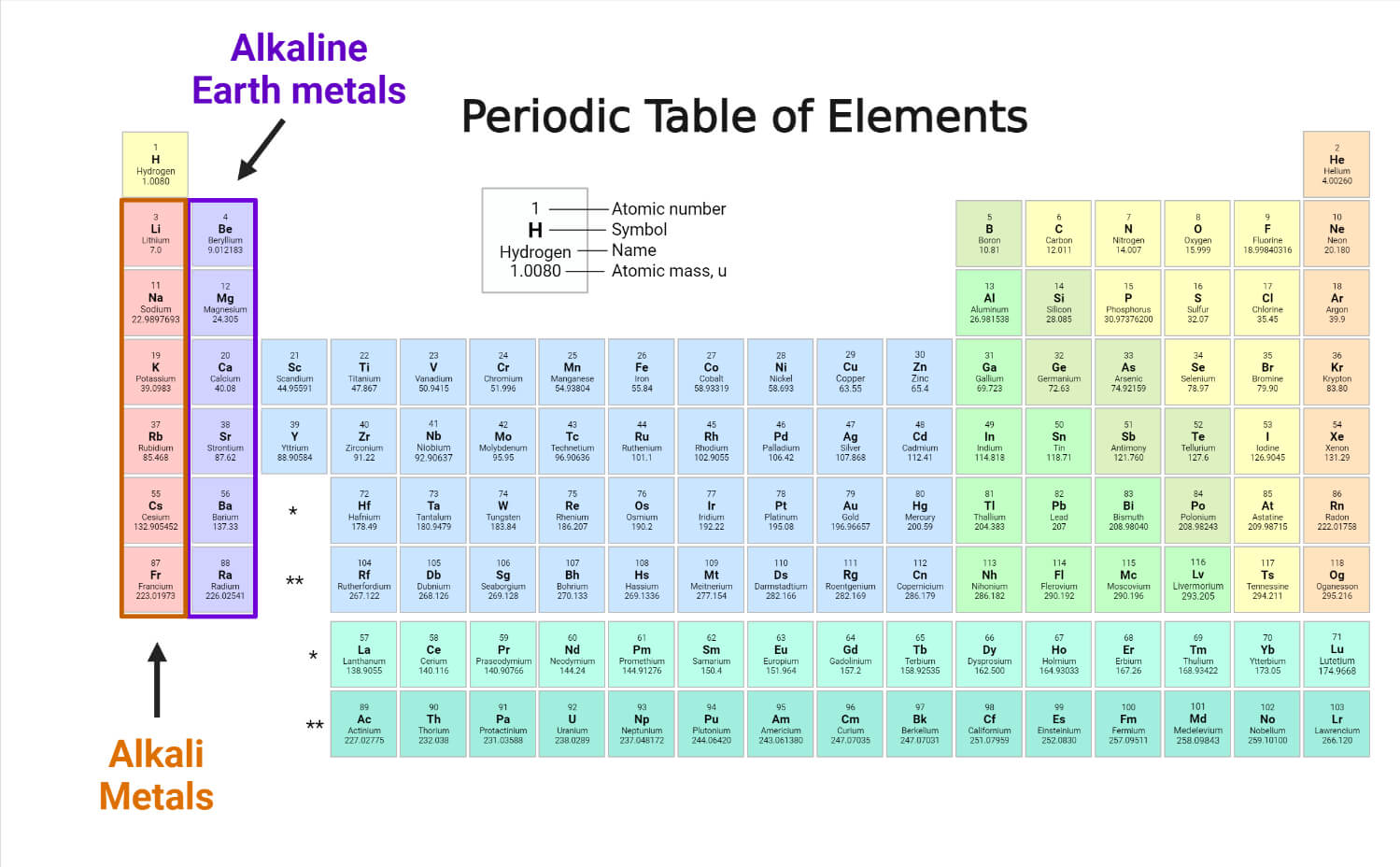
Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass . chemistry chapter 5. 1) the modern periodic law states that the properties of the elements repeat when the periodic. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? because of their higher positive charge (+2) and smaller ionic radii, the alkaline earth metals have a much greater tendency to form. (i) it is solid at room temperature. all of the following properties of the alkaline earth metals increase going down the group except (a) atomic radius (b) first ionization. The alkaline earth metals tend to form +2. alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. alkaline earths have low electron affinities and low electronegativities. (ii) it easily forms an oxide when exposed to air. As with the alkali metals, the properties depend on. 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. consider the following properties of an element:
from scienceinfo.com
which of the following properties of the alkaline earth metals decreases with increasing atomic weight? The alkaline earth metals tend to form +2. 1) the modern periodic law states that the properties of the elements repeat when the periodic. chemistry chapter 5. alkaline earths have low electron affinities and low electronegativities. (i) it is solid at room temperature. consider the following properties of an element: 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. As with the alkali metals, the properties depend on. all of the following properties of the alkaline earth metals increase going down the group except (a) atomic radius (b) first ionization.
Comparison of properties of Alkali and Alkaline Earth Metals
Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass The alkaline earth metals tend to form +2. As with the alkali metals, the properties depend on. all of the following properties of the alkaline earth metals increase going down the group except (a) atomic radius (b) first ionization. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? chemistry chapter 5. 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. because of their higher positive charge (+2) and smaller ionic radii, the alkaline earth metals have a much greater tendency to form. (i) it is solid at room temperature. 1) the modern periodic law states that the properties of the elements repeat when the periodic. alkaline earths have low electron affinities and low electronegativities. The alkaline earth metals tend to form +2. (ii) it easily forms an oxide when exposed to air. consider the following properties of an element:
From brokeasshome.com
Where To Find Atomic Mass In Periodic Table Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass (ii) it easily forms an oxide when exposed to air. alkaline earths have low electron affinities and low electronegativities. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? (i) it is solid at room temperature. chemistry chapter 5. 1) the modern periodic law states that the properties of the elements repeat. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From www.expii.com
Alkali Metals — Overview & Properties Expii Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass which of the following properties of the alkaline earth metals decreases with increasing atomic weight? alkaline earths have low electron affinities and low electronegativities. alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. (ii) it easily forms an oxide when exposed to air. 5.0 (4 reviews) in the periodic table, there. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From scienceinfo.com
Comparison of properties of Alkali and Alkaline Earth Metals Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass because of their higher positive charge (+2) and smaller ionic radii, the alkaline earth metals have a much greater tendency to form. chemistry chapter 5. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? (i) it is solid at room temperature. consider the following properties of an element: 1) the. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From www.thoughtco.com
What Are the Properties of the Alkaline Earth Metals? Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass because of their higher positive charge (+2) and smaller ionic radii, the alkaline earth metals have a much greater tendency to form. As with the alkali metals, the properties depend on. consider the following properties of an element: alkaline earths have low electron affinities and low electronegativities. 1) the modern periodic law states that the properties of. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From thechemistrynotes.com
Trends of the Properties of Group 2 (Alkaline Earth) Metals Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass all of the following properties of the alkaline earth metals increase going down the group except (a) atomic radius (b) first ionization. consider the following properties of an element: (i) it is solid at room temperature. (ii) it easily forms an oxide when exposed to air. As with the alkali metals, the properties depend on. chemistry chapter. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From www.slideserve.com
PPT Elements and The Periodic Table PowerPoint Presentation, free download ID215276 Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass which of the following properties of the alkaline earth metals decreases with increasing atomic weight? The alkaline earth metals tend to form +2. 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. (ii) it easily forms an oxide when exposed to air. chemistry chapter 5. (i) it is solid at room temperature. consider. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From byjus.com
Alkaline Earth Metals Occurrence and Extraction,Physical Properties Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass alkaline earths have low electron affinities and low electronegativities. The alkaline earth metals tend to form +2. 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. 1) the modern periodic law states that the properties of the elements repeat. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From www.slideserve.com
PPT Elements and the Periodic Table PowerPoint Presentation, free download ID8779622 Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass chemistry chapter 5. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. (ii) it easily forms an oxide when exposed to air. all of the following properties of the alkaline earth metals increase going down the group except. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From www.slideserve.com
PPT Elements and their Properties PowerPoint Presentation, free download ID6909242 Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. The alkaline earth metals tend to form +2. 1) the modern periodic law states that the properties of the elements repeat when the periodic. chemistry chapter 5. (ii) it easily forms an oxide when exposed to air. alkaline earths have low electron affinities and low. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From pediaa.com
Difference Between Alkali Metals and Alkaline Earth Metals Definition, Properties, Examples Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass alkaline earths have low electron affinities and low electronegativities. chemistry chapter 5. alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. The alkaline earth metals tend to form +2. (i) it is solid at room temperature. because. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From xlskoor.blogspot.com
Alkali Metals Chemistry Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass As with the alkali metals, the properties depend on. consider the following properties of an element: 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? alkaline earths have low electron affinities and low electronegativities. (ii) it easily forms. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From ar.inspiredpencil.com
Periodic Table Of Elements Alkaline Earth Metals Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass As with the alkali metals, the properties depend on. chemistry chapter 5. because of their higher positive charge (+2) and smaller ionic radii, the alkaline earth metals have a much greater tendency to form. 1) the modern periodic law states that the properties of the elements repeat when the periodic. The alkaline earth metals tend to form +2.. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From utedzz.blogspot.com
Alkaline Earth Metals Periodic Table Definition Periodic Table Timeline Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass As with the alkali metals, the properties depend on. alkaline earths have low electron affinities and low electronegativities. consider the following properties of an element: 1) the modern periodic law states that the properties of the elements repeat when the periodic. (ii) it easily forms an oxide when exposed to air. 5.0 (4 reviews) in the periodic table,. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From slideplayer.com
The Periodic Law (Periodic Table) ppt download Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass alkaline earths have low electron affinities and low electronegativities. As with the alkali metals, the properties depend on. (ii) it easily forms an oxide when exposed to air. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? alkaline earth metals are good reducing agents that tend to form the +2 oxidation. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From overallscience.com
Trends in atomic and physical properties of alkali metals Overall Science Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass alkaline earths have low electron affinities and low electronegativities. (ii) it easily forms an oxide when exposed to air. all of the following properties of the alkaline earth metals increase going down the group except (a) atomic radius (b) first ionization. because of their higher positive charge (+2) and smaller ionic radii, the alkaline earth metals have. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From www.vrogue.co
Alkali Metals Periodic Table Properties Elcho Table vrogue.co Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline earth metals tend to form +2. alkaline earths have low electron affinities and low electronegativities. 5.0 (4 reviews) in the periodic table, there is a periodic pattern in. consider the following properties of an element: all of the following. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From byjus.com
Alkali Metals Properties, Electronic Configuration, Periodic Trends & Uses Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass because of their higher positive charge (+2) and smaller ionic radii, the alkaline earth metals have a much greater tendency to form. (ii) it easily forms an oxide when exposed to air. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? The alkaline earth metals tend to form +2. 5.0 (4 reviews). Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.
From ashleyrush2.weebly.com
AlkalineEarth Metals Periodic Table Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass (i) it is solid at room temperature. alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. chemistry chapter 5. The alkaline earth metals tend to form +2. which of the following properties of the alkaline earth metals decreases with increasing atomic weight? As with the alkali metals, the properties depend on.. Which Of The Following Properties Of Alkaline Earth Metals Decreases With Increasing Atomic Mass.